2014
July 30, 2014
Kurita Develops High-Performance, Low-Cost Purified Water Production System for Pharmaceutical Manufacturing Process
Water Treatment System Developed in Pursuit of High Quality and High Level of Safety by Blocking Proliferation of Living Microbes
Kurita Water Industries Ltd. (Head Office: Nakano-ku, Tokyo; President: Toshiyuki Nakai) has developed and begun sale of a purified water production system highly effective for blocking proliferation of living microbes and that improves the quality of water used in manufacturing pharmaceuticals. The new system keeps living microbes in it at an extremely low number. It does not need an activated carbon filter, which causes generation of microbes, because it uses a special proprietarily developed UV irradiator. The system also features a simple device configuration that permits lower initial cost. Kurita will promote application of this system in the pharmaceutical industry and respond to a wide range of customer needs for higher product quality and lower cost.
The pharmaceutical manufacturing process requires a high level of quality control corresponding to the validation*. Purified water is used as a raw material of pharmaceutical products and as cleaning water for devices and instruments. It is produced by removing ionic components and impurities from raw water with a system that combines technologies such as ion exchange and membrane filtration. Appropriate control of living microbes is essential for ensuring stable quality of purified water. Therefore, customers who pursue high quality and a high level of safety in their products have strong demand for enhanced control of microbes in the overall purified water production system, with the risk of microbe proliferation controlled thoroughly in each component device as well as at the system’s outlet.
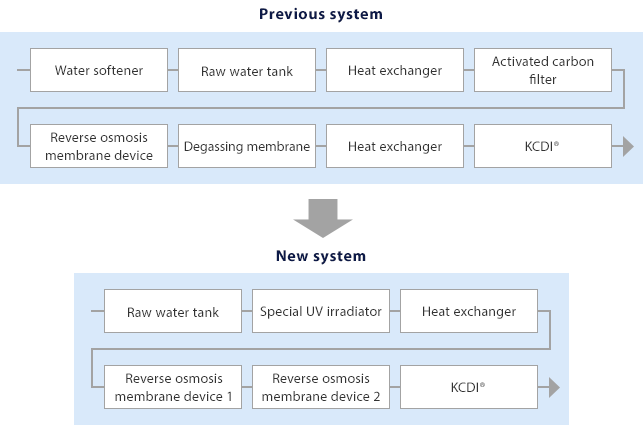
In response, Kurita enabled practical application of a system, ahead of other companies, that sterilizes all component devices and pipes intermittently with water at 80℃ or higher and has been offering the system widely. This latest water treatment system developed produces water with a high level of purity through ultraviolet sterilization and reverse osmosis membrane treatment and then by using a continuous electric deionizer (KCDI®). It also applies for the first time a special UV irradiator with high sterilization performance so it does not need an activated carbon filter, which forms a hotbed for microbes. For this system, Kurita has combined the existing hot water sterilization with UV sterilization and succeeded in ensuring a high sterilization effect, which reduces the number of living microbes in the system to a virtually undetectable level (one microbe/ml or less). And for residual chlorine that causes oxidation degradation of KCDI®, the special UV irradiator removes not only free chlorine but also combined chlorine, contributing to maintenance of the system's performance and stable operation.
The system's device configuration is simpler than those of previous systems because it has eliminated components such as water softener and degassing membrane, as well as activated carbon filters. This permits a 20% reduction in the initial cost for introducing the system and a 50% reduction in the time it takes to complete hot water sterilization, which was about six hours. The system also requires little space because it is standardized. Site installation takes less time than previous systems because validation and test operation are undertaken at Kurita's plant prior to installation.
For more than 30 years, Kurita has provided the pharmaceutical industry with numerous systems for producing sterile purified water and injection solvents, as well as purified water. The company boasts one of the largest market shares of such systems in Japan. Moving forward, Kurita will position this purified water production system as one of its strategic products to cultivate the domestic market more deeply. At the same time, it will view a wider range of customer needs, including those in other countries where pharmaceutical markets are expected to grow.
- *Validation
Validation refers to a statutory standard for manufacturing and installation of equipment for manufacturing pharmaceutical products and medicines. It requires that scientific validation of whether the equipment, its performance, procedures, process flow, quality control method, and other elements reach the levels required by the standard, as well as recording and documentation of inspection data related to quality and safety of pharmaceutical products, should be undertaken in the phases of design, manufacturing, and test operation. - *Supplementary materials: Purified Water Production System for the Process of Manufacturing Pharmaceutical Products (System Flow and Appearance)
Purified Water Production System for Process of Manufacturing Pharmaceutical Products (System Flow and Appearance)
- System flow (device configuration)
The number of devices, which was eight with previous systems, was reduced to six for simpler flow.
Main devices
♦Special UV irradiator
- Ultraviolet irradiator boasting superior sterilization performance
- It also prevents oxidation degradation of devices in the following stages since it is capable of removing not only free but also combined chlorine, which cannot be removed with activated carbon.
♦Use of reverse osmosis membrane devices in two stages
- Reverse osmosis membrane devices are used in two consecutive stages for effective removal of ions, salt, and other components. This configuration means the new system does not need equipment such as water softener and degassing membrane, which makes it a space-saving system.
♦KCDI (continuous electric deionizer)
- Because KDCI is an electric deionizer, chemical treatment is not necessary. The deionizer can be operated continuously, ensuring stable quality of purified water the system produces.
- Appearance of the system